Paracetamol Poisoning
Introduction:
Paracetamol (Acetaminophen) has the chemical name N-acetyl-p-aminophenol abbreviated (APAP) and the chemical formula C8H9NO2. [1]
Paracetamol Structure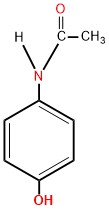
Paracetamol is one of the most consumed over the counter (OTC) drugs for its analgesic and antipyretic effects and it is available alone or in combination with other drugs for pain relief, fever, flu, cold, cough and allergy [2]. Paracetamol (Acetaminophen) is the active metabolite of phenacetin, and it is a weak inhibitor of the enzymes cyclo-oxygenase1 (COX-1) and cyclo-oxygenase2 (COX-2) in peripheral tissues, which are involved in inflammatory reactions [3].
As an analgesic and antipyretic drug, Paracetamol is equivalent to aspirin but lacks aspirin’s anti-inflammatory, platelets-inhibiting properties and does not raise blood uric acid levels [3].
Paracetamol exerts its pain relieving pharmacological effects by acting primarily on COX-1, COX-2 and COX-3 enzymes isoforms in the Central Nervous System (CNS), which are involved in Prostaglandin synthesis thus raising the pain threshold, and its antipyretic effect is due to direct effect on heat-regulating centres of Hypothalamus [1].
After oral administration of Paracetamol, peak blood concentrations reached in 30-60 minutes, and the half-life of Paracetamol (Acetaminophen) is 2-3 hours (3). Paracetamol is safe if taken in therapeutic doses with nearly no side effects [1] but if taken in excess it could cause liver damage.
Therapeutic dose of Paracetamol:
1- 500 to 1000 mg every four to six hours for adults and children 12 years and over as necessary, with a maximum of 4000 mg in any 24 hour period.
2- 15 mg per kg every four to six hours for children (1 month to 12 years), which can be given as required, with no more than four doses in 24 hours [4].
Paracetamol (Acetaminophen) Drug Metabolism:
In general, drugs in the human body undergo biotransformation to be excreted outside the body (Detoxification). Major biotransformation called Phase I and Phase II reactions. Phase I reactions convert parent drug to inactive drug metabolite or sometimes to a more active metabolite, and this is done by introducing or unmasking drugs’ functional group (-OH, -NH, -SH). Phase II reactions transform Phase I drug metabolites or a drug to a highly polar inactive drug conjugates with glucuronic acid, sulfuric acid, acetic acid, Glutathione or amino acid, which are easily excreted outside the body. Some drugs undergo Phase II reactions then Phase I reactions. The liver is the main site of drug biotransformation [5].
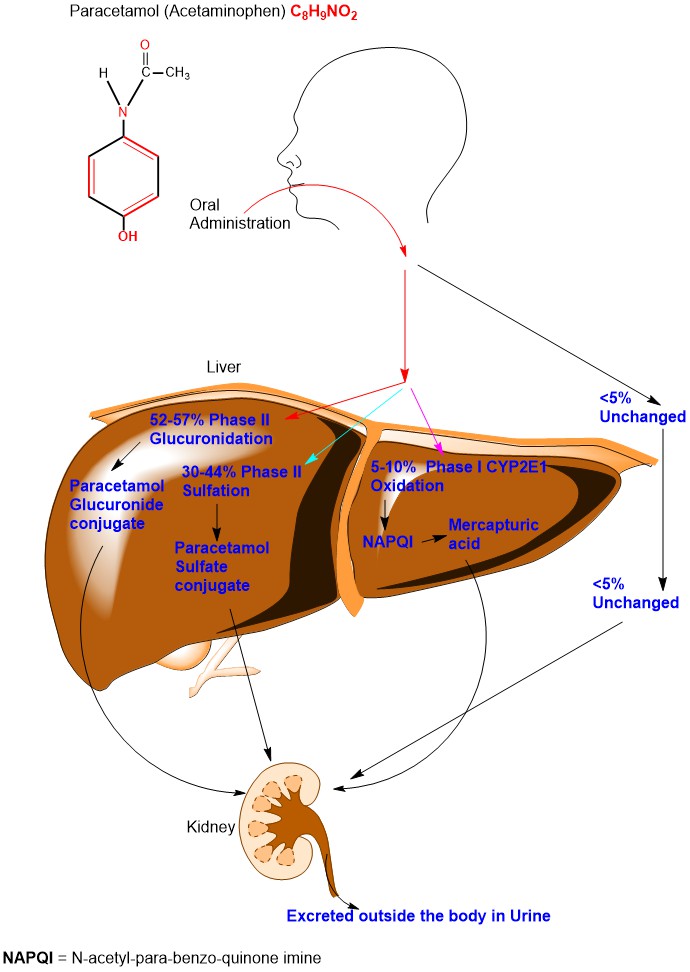
After oral administration of Paracetamol, 52-57% undergoes Phase II Glucuronidation, 30-44% undergo Phase II Sulfation, 5-10% undergoes Phase I reaction and less than 5% excreted unchanged in the urine [6]. (Appendix 1)
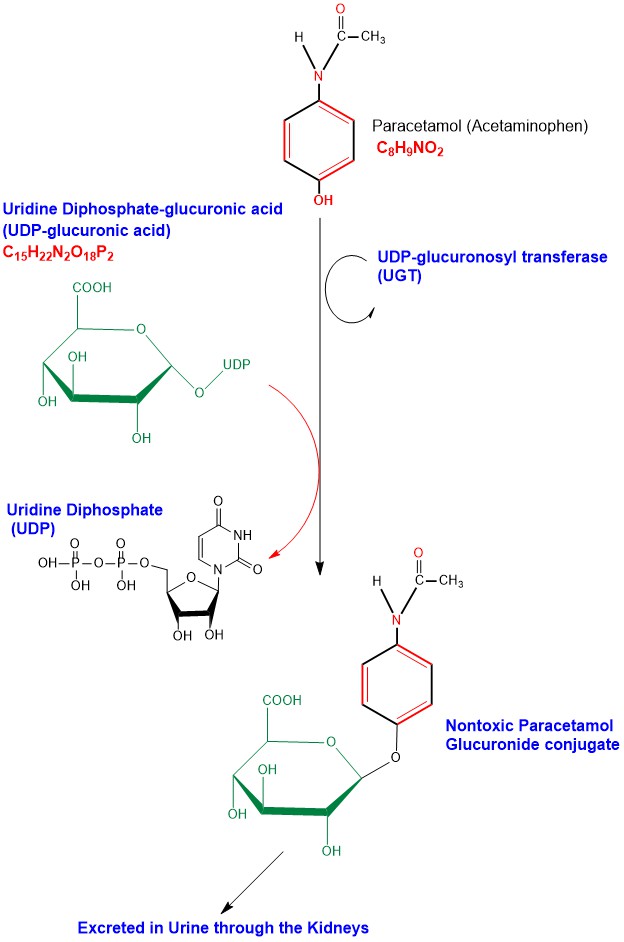
Glucuronidation of Paracetamol takes place in liver cells (Hepatocytes) microsomes, where a glucuronosyl group is transferred from Uridine Diphosphate-glucuronic acid (UDP-glucuronic acid) to Paracetamol, the reaction is catalyzed by the enzyme UDP-glucuronosyl transferase (UGT), forming the nontoxic inactive water soluble glucuronide conjugate (Paracetamol Glucuronide) to be excreted outside the body in urine through the kidneys [6]. (Appendix 2)
Sulfation of Paracetamol in Hepatocytes catalyzed by the cytosolic enzyme sulfotransferase transferring a sulfate group from 3'-phosphoadenosine 5'-phosphosulfate (PAPS) to Paracetamol forming the nontoxic inactive water-soluble sulfate conjugate (Paracetamol Sulfate) to be excreted outside the body in urine through the kidneys [6]. (Appendix 3)
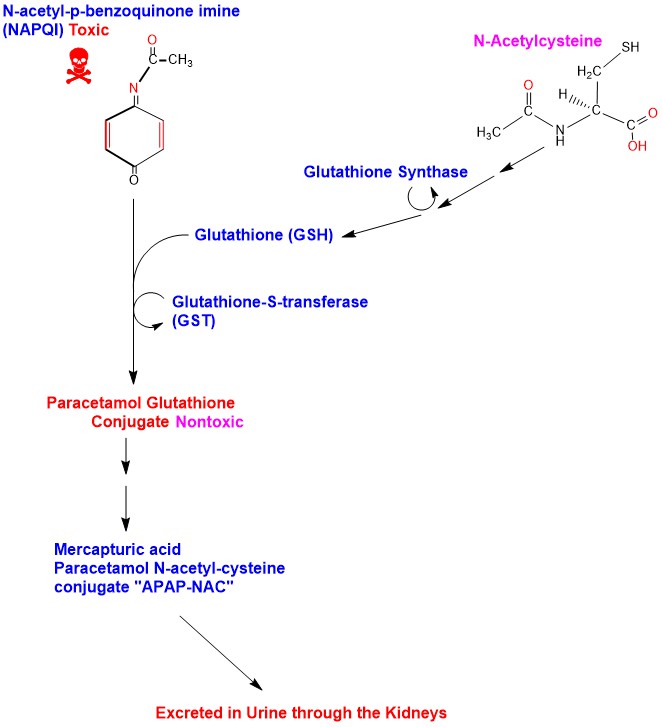
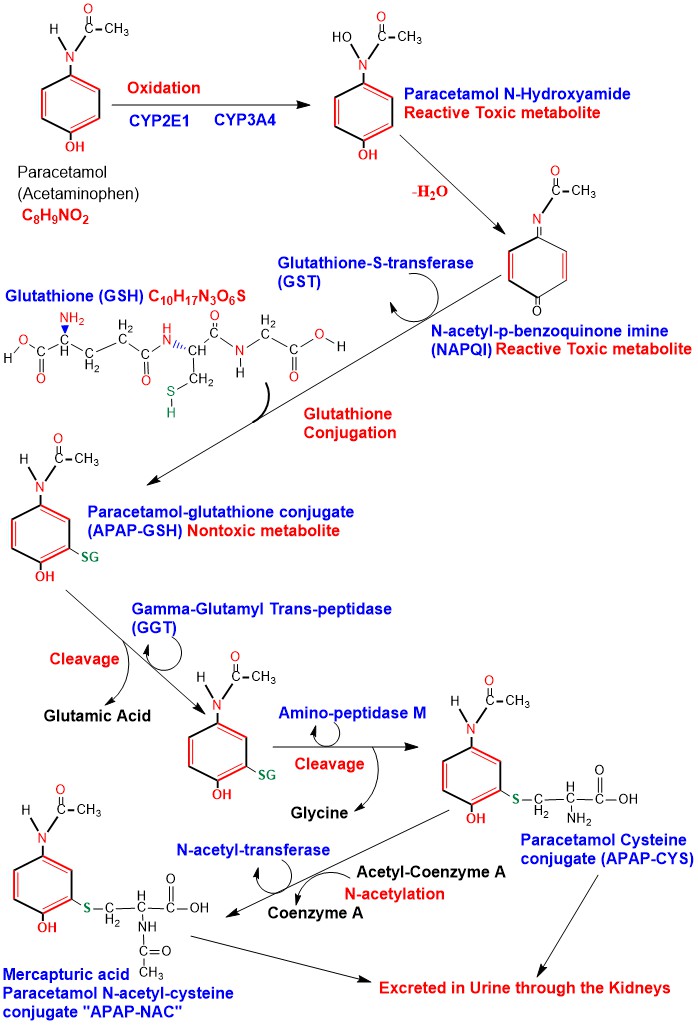
Phase I oxidation of Paracetamol is carried out in the Hepatocytes Cytochrome P450 (CYP) system mainly CYP2E1 and to a lesser extent CYP1A2 and CYP3A4, producing the toxic active metabolite N-acetyl-p-benzoquinone imine (NAPQI). When Paracetamol is taken within therapeutic doses, NAPQI is conjugated with Glutathione forming Paracetamol-glutathione conjugate (APAP-GSH) and the reaction is catalyzed by the enzyme glutathione-S-transferase (GST), rendering it nontoxic [6]. Glutathione is a tripeptide consisting of the amino acids cysteine, glutamic acid and glycine. Amino acids of Glutathione in the APAP-GSH conjugate undergo sequential cleavage of glutamic acid catalyzed by the enzyme Gamma-Glutamyl Trans-peptidase (GGT), then cleavage of glycine, catalyzed by the enzyme Amino-peptidase M producing Paracetamol Cysteine conjugate (APAP-CYS) to be excreted in urine. APAP-CYS undergoes N-acetylation catalyzed by the enzyme N-acetyl-transferase, transferring acetyl group from acetyl-coenzyme A to APAP-CYS producing Mercapturic acid (Paracetamol N-acetyl-cysteine conjugate) “APAP-NAC” which is excreted in urine [7] [8]. (Appendix 4)
Paracetamol Poisoning (Liver Toxicity) Mechanism:
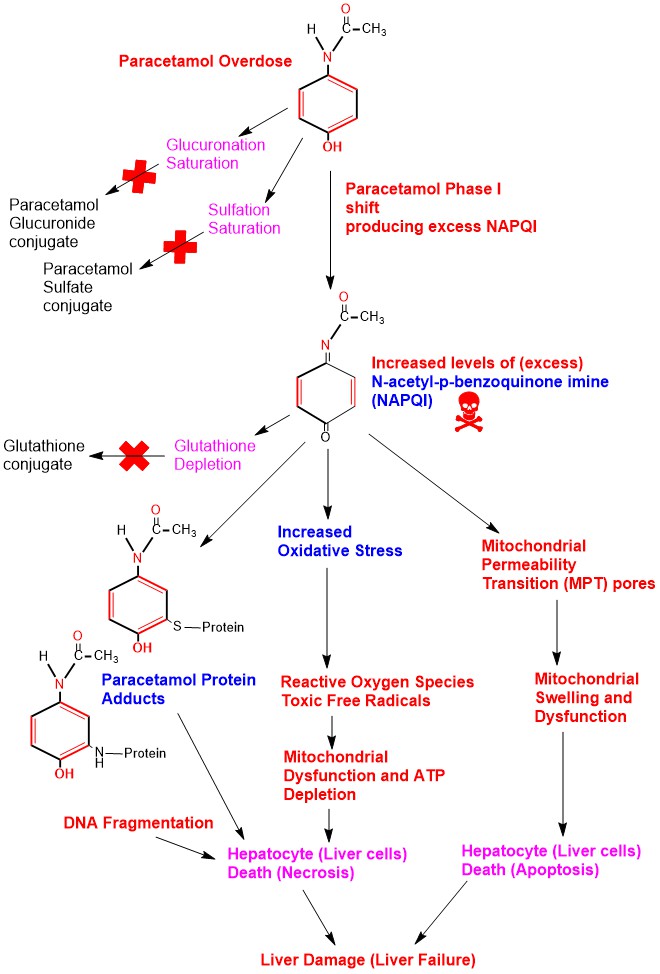
Paracetamol Hepatic (liver) Toxicity (damage) occurs when Paracetamol taken as an acute oral dose exceeding 150 mg/kg in children and 7.5 grams in adults within 24 hours [9]. Acutely increased levels of Paracetamol in the body will saturate Phase II glucuronidation and sulfation detoxification pathways in the liver shifting Paracetamol to Phase I oxidation producing high levels of NAPQI. Excess NAPQI overwhelms glutathione conjugation pathway leading to glutathione stores depletion in the liver. Excess NAPQI leads to:
- Increased oxidative stress.
- Formation of toxic free radicals (Reactive Oxygen Species, superoxide, peroxynitrite).
- Binds to cellular proteins especially in the mitochondria forming Paracetamol protein adducts leading to DNA fragmentation particularly in the mitochondria.
All these factors lead to mitochondrial dysfunction and reduced Adenosine Triphosphate (ATP) production and stores depletion causing cell death (necrosis) . Another factor is opening of Mitochondrial Permeability Transition (MPT) pores leading to mitochondrial swelling and finally hepatocytes apoptosis [10]. (Appendix 5)
Clinical Paracetamol Poisoning:
Timing of ingestion of the toxic dose is important, and clinically Paracetamol poisoning divided into 4 stages [9].
| Stage | Elapsed time after toxic dose ingestion | Symptoms, signs and findings |
| 1 | 0-24 h | Anorexia, nausea and vomiting |
| 2 | 24-72 h | Right upper guardant abdominal pain, elevated liver enzymes and bilirubin |
| 3 | 72-96 h | Vomiting and symptoms of liver failure. Sometimes renal failure and pancreatitis |
| 4 | >5 days | Resolution of hepatotoxicity or progression to multiple organ failure |
Treatment is with oral or I.V. N-acetylcysteine administration as soon as possible to replenish glutathione stores in the liver to detoxify NPQI. Oral Activated charcoal to prevent further Paracetamol absorption from the gut [9]. (Appendix 6)
References:
1- Acetaminophen, Drug Bank, Drug created on June 13, 2005 / Updated on February 09, 2017 https://www.drugbank.ca/drugs/DB00316
2- Acetaminophen Information, U.S. Food & Drug Administration http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm165107.htm
3- Bertman G. Katzung, Anthony J. Trevor , Basic & Clinical Pharmacology 13th Edition 2015, page 634.
4- Recommended Paracetamol doses, Australian Gov. Dept. of Health https://www.tga.gov.au/community-qa/recommended-paracetamol-doses
5- Bertman G. Katzung, Anthony J. Trevor , Basic & Clinical Pharmacology 13th Edition 2015, page 57.
6- Liudmila Mazaleuskaya, Katrin Sangkuhl, Caroline Thorn, Garret FitzGerald, Russ Altman, Teri Klein, Acetaminophen Pathway (toxic doses), Pharmacokinetics https://www.pharmgkb.org/pathway/PA166117881
7- Bertman G. Katzung, Anthony J. Trevor , Basic & Clinical Pharmacology 13th Edition 2015, page 65.
8- John Stephen Forte BPharm (Hons), MSc (Lond), Paracetamol safety versus toxicity http://www.mcppnet.org/publications/issue06-6.pdf
9- Gerald F. O’Malley, DO, Thomas Jefferson University and Hospital ; Rika O’Malley, MDA, Acute Acetaminophen Poisoning, Merck Manual. http://www.merckmanuals.com/professional/injuries-poisoning/poisoning/acetaminophen-poisoning
10- Acetaminophen induced Hepatotoxicity: a comprehensive update https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4913076/
Appendices:
Appendix1: Diagrammatic illustration of Paracetamol Metabolism.
Appendix2: Illustration of Paracetamol Phase II Glucuronidation detoxification chemical reactions steps.
Appendix3: Illustration of Paracetamol Phase II Sulfation detoxification chemical reactions steps.
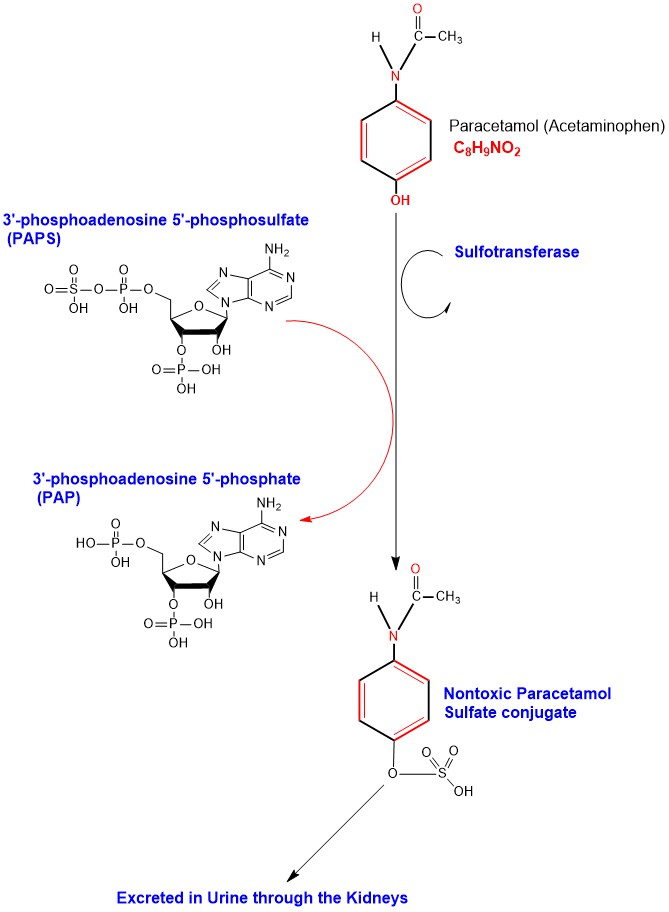
Appendix4: Illustration of Paracetamol Phase I oxidation to NAPQI and its detoxification.
Appendix5: Illustration of Hepatotoxicity in Paracetamol Poisoning.
Appendix6: N-acetylcysteine action in Paracetamol Poisoning.